CBSE Class 12 Chemical Kinetics Multiple Choice Questions with Answers. MCQ Questions Class 12 Chemical Kinetics with Answers Is Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Chemical Kinetics MCQs with Answers to know their preparation level.
Students who are searching for NCERT MCQ Questions for Class 12 Chemical Kinetics with Answers are compiled here to get good practice on all fundamentals. Know your preparation level on MCQ Questions for Class 12 Chemical Kinetics with Answers. You can also verify your answers from our provided MCQ Class 12 Chemical Kinetics with Answers. So, ace up your preparation with MCQ of Class 12 Chemistry Examinations.
MCQ Questions Class 12 Chemical Kinetics with Answers - Set - 4
Question 1:
By increase in temperature by 10 K, the rate of reaction becomes double. How many times the rate of reaction will be if the temperature is increased from 303K to 353 K?
(a) 4
(b) 8
(c) 16
(d) 32
Correct Answer – (D)
Question 2 :
The activation energy of exothermic reaction A ➔ B 80 kJ mol-1. The heat of reaction is 200 kJ mol-1. The activation energy for the reaction B ➔ A (in kJ mol-1) will be
(a) 80
(b) 120
(c) 40
(d) 280
Correct Answer – (D)
Question 3 :
The rate of a chemical reaction doubles for every 10°C rise of temperature. If the temperature is raised by 50°C., the rate of the reaction increases by about
(a) IO times
(b) 24 times
(c) 32 times
(d) 64 times
Correct Answer – (C)
Question 4 :
N2 (g) + 2H2 (g) ⇌ 2NH3 (g) + 22 kcal
The activation energy for the forward reaction is 50 kcal. What is the activation energy for the backward reaction?
(a) 72 kcal
(c) -72 kcal
(b) 28 kcal
(d) -28kcal
Correct Answer – (A)
Question 5 :
A chemical reaction was carried out at 300 K and 280 K. The rate constants were found to be k1 and k2 respectively. Then
(a) k2 ≈ 0.25 k1
(b) k2 ≈0.5 k1
(c) k2 ≈ 4 k1
(d) k2 ≈ 2 k1
Correct Answer – (A)
MCQ Questions Class 12 Chemical Kinetics with Answers
Question 6:
In the given graph the activation energy, Ea for the reverse reaction will be
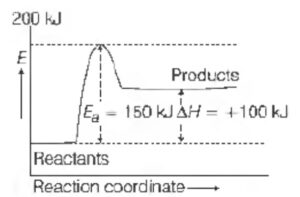
(a) 50 kJ
(b) 50 kJ
(c) 200 kJ
(d) 100 kJ
Correct Answer – (B)
Question 7:
The activation energy of a reaction at a given temperature is found to be 2.303 RT J mol-1. The ratio of rate constant to the Arrhenius factor is
(a) 0.01
(b) 0.1
(c) 0.02
(d) 0.001
Correct Answer – (B)
Question 8:
A given sample of milk turns sour at room temperature (27°C) in 5 h. In a refrigerator at -3° C., it can be stored 10 times longer. The energy of activation for the souring of milk is
(a) 2.303 x 5 R kJ mol-1
(b) 2.303 x 3 R kJ mol-1
(c) 2.303 x 2.7 R kJ mol-1
(d) 2.303 x 10 R kl mol-1
Correct Answer – (C)
Question 9:
The rate of a reaction is doubled for every 10° rise in temperature. The increase in reaction rate as a result of temperature rise from 10° to 100° is
(a) 112
(b) 512
(c) 400
(d) 614
Correct Answer – (B)
Question 10:
For a reaction taking place in three steps, the rate constants are k1 , k2 and k3 and overall rate constant is k = k1k3 / k2 . If the energies of activation E1, E2 and E3 are 60, 30 and 10 kJ mol-1 , respectively, then the overall energy of activation is
(a) 30 kJ mol-1
(b) 40 kJ mol-1
(c) 60 kJ mol-1
(d) l00 kJ mol-1
Correct Answer – (A)
- NCERT Solutions Class 11 Chemistry Chapter 1 : Some Basic Concepts of Chemistry
- NCERT Solutions Class 11 Chemistry Chapter 2 : Structure Of The Atom
- NCERT Solutions Class 11 Chemistry Chapter 3 : Classification of Elements and Periodicity in Properties
- NCERT Solutions Class 11 Chemistry Chapter 4 : Chemical Bonding and Molecular Structure
- NCERT Solutions Class 11 Chemistry Chapter 5 : States of Matter
- NCERT Solutions Class 11 Chemistry Chapter 6 : Thermodynamics
- NCERT Solutions Class 11 Chemistry Chapter 7 : Equilibrium
- NCERT Solutions Class 11 Chemistry Chapter 8 : Redox Reactions
- NCERT Solutions Class 11 Chemistry Chapter 9 : Hydrogen
- NCERT Solutions Class 11 Chemistry Chapter 10 : The s-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 11 : The p-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 12 : Organic Chemistry: Some Basic Principles and Techniques
- NCERT Solutions Class 11 Chemistry Chapter 13 : Hydrocarbons
- NCERT Solutions Class 11 Chemistry Chapter 14 : Environmental Chemistry



