CBSE Class 12 Surface Chemistry Multiple Choice Questions with Answers. MCQ Questions Class 12 Surface Chemistry with Answers Is Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Surface Chemistry MCQs with Answers to know their preparation level.
Students who are searching for NCERT MCQ Questions for Class 12 Surface Chemistry with Answers are compiled here to get good practice on all fundamentals. Know your preparation level on MCQ Questions for Class 12 Surface Chemistry with Answers. You can also verify your answers from our provided MCQ Class 12 Surface Chemistry with Answers. So, ace up your preparation with MCQ of Class 12 Chemistry Examinations.
MCQ Questions Class 12 Surface Chemistry with Answers - Set - 2
Question 1:
Peptisation involves
(a) precipitation of colloidal particles
(b) disintegration of colloidal aggregates
(c) evaporation of dispersion medium
(d) Impact of molecules of the dispersion medium on the colloidal particles
Correct Answer – (B)
Question 2 :
Which of the following reactions is an example of heterogeneous catalysis?
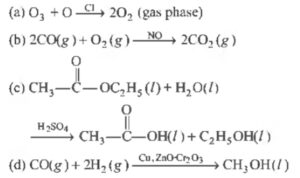
Correct Answer – (D)
Question 3 :
Identify the correct statement regarding enzymes.
(a) Enzymes are specific biological catalysts that can normally function at very high temperatures (T – 1000 K)
(b) Enzymes are normally heterogeneous catalysts that are very specific in their action
(c) Enzymes are specific biological catalysts that cannot be poisoned
(d) Enzymes are specific biological catalysts that possess well defined active sites
Correct Answer – (D)
Question 4 :
A catalyst
(a) lowers the activation energy
(b) changes the rate constant
(c) changes the product
(d) itself destroys in the reaction
Correct Answer – (A)
Question 5 :
In a chemical reaction, catalyst
(a) decreases the energy of activation
(b) increases the energy of activation
(c) does not change energy of activation
(d) None of the above
Correct Answer – (A)
MCQ Questions Class 12 Surface Chemistry with Answers
Question 6:
Formation of ammonia from H2 and N2 by Haber’s process using Fe is an example of
(a) heterogeneous catalysis
(b) homogeneous catalysis
(c) enzyme catalysis
(d) non-catalytic process
Correct Answer – (A)
Question 7:
Active charcoal is a good catalyst because
(a) made up of carbon atoms
(b) is very reactive
(c) has more adsorption power
(d) has inert nature toward reagent
Correct Answer – (C)
Question 8:
Catalyst in a reaction
(a) lowers the activation energy
(b) increases the rate of reaction
(c) Both (a) and (b)
(d) initiates the reaction
Correct Answer – (C)
Question 9:
Assertion (A) Catalyst increases the rate of a reaction.
Reason (R) In presence of a catalyst, the activation energy of the reaction increases. The correct answer is
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are correct but R is not the correct explanation of A
(c) A is correct but R is incorrect
(d) Assertion is not correct, but Reason is correct
Correct Answer – (C)
Question 10:
Which of the following reaction requires catalyst?
(a) S + O2 → SO2
(b) C + O2 → CO2
(c) 2SO2 + O2 → 2SO3
(d) All of the above
Correct Answer – (C)
- NCERT Solutions Class 11 Chemistry Chapter 1 : Some Basic Concepts of Chemistry
- NCERT Solutions Class 11 Chemistry Chapter 2 : Structure Of The Atom
- NCERT Solutions Class 11 Chemistry Chapter 3 : Classification of Elements and Periodicity in Properties
- NCERT Solutions Class 11 Chemistry Chapter 4 : Chemical Bonding and Molecular Structure
- NCERT Solutions Class 11 Chemistry Chapter 5 : States of Matter
- NCERT Solutions Class 11 Chemistry Chapter 6 : Thermodynamics
- NCERT Solutions Class 11 Chemistry Chapter 7 : Equilibrium
- NCERT Solutions Class 11 Chemistry Chapter 8 : Redox Reactions
- NCERT Solutions Class 11 Chemistry Chapter 9 : Hydrogen
- NCERT Solutions Class 11 Chemistry Chapter 10 : The s-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 11 : The p-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 12 : Organic Chemistry: Some Basic Principles and Techniques
- NCERT Solutions Class 11 Chemistry Chapter 13 : Hydrocarbons
- NCERT Solutions Class 11 Chemistry Chapter 14 : Environmental Chemistry