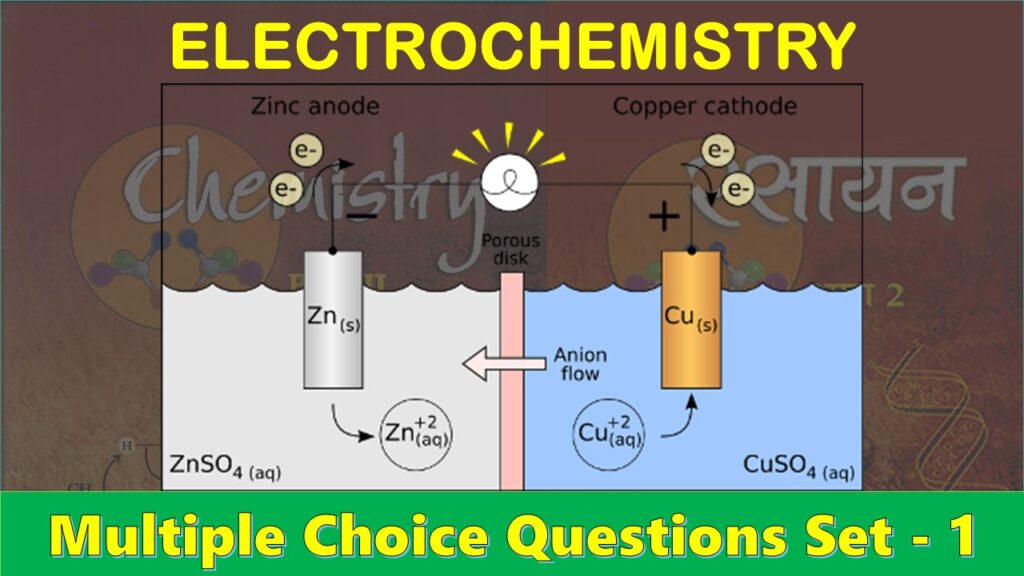
CBSE Class 12 Electrochemistry Multiple Choice Questions with Answers. MCQ Questions Class 12 Electrochemistry with Answers Is Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Electrochemistry MCQs with Answers to know their preparation level.
Students who are searching for NCERT MCQ Questions for Class 12 Electrochemistry with Answers are compiled here to get good practice on all fundamentals. Know your preparation level on MCQ Questions for Class 12 Electrochemistry with Answers. You can also verify your answers from our provided MCQ Class 12 Electrochemistry with Answers. So, ace up your preparation with MCQ of Class 12 Chemistry Examinations.
MCQ Questions Class 12 Electrochemistry with Answers - Set - 1
Question 1:
The e.m.f. of the cell Zn/Zn2+ (0.01 M) || Fe2+ (0.001 M) Fe at 298 K is 0.2905 volt. Then the value of equilibrium constant for the cell reaction is:
(a) e0.32/0.0295
(b) 100.32/0.0295
(c) 100.26/0.0295
(d) 100.32/0.0591
Correct Answer – (A)
Question 2 :
The standard reduction potentials of X, Y, Z metals are 0.52, -3.03, -1.18 respectively. The order of reducing power of the corresponding metals is:
(a) Y > Z > X
(b) X > Y > Z
(c) Z > Y > X
(d) Z > X > Y
Correct Answer – (A)
Question 3 :
The Standard electrode potentials for the half cell reactions are as follows
Zn ? Zn2+ + 2e– [E° = 0.41 V]
Fe ? Fe2+ + 2e– [E° = 0.76 V]
(a) -0.35 V
(b) 0.35 V
(c) + 1.17 V
(d) -1.17 V
Correct Answer – (B)
Question 4 :
How many coulombs are required for the oxidation of 1 mole of H2O to O2?
(a) 1.93 × 105 C
(b) 9.65 × 104 C
(c) 3.86 × 105 C
(d) 4.825 × 105 C
Correct Answer – (A)
Question 5 :
For the electro-chemical cell:
M| M+|| X–| X, E0M+,M = 0.44 V and E0x,x– = 0.33 V
From the data one can deduce that
(a) M+ X → M+ + X– is the spontaneous change
(b) M+ + X– → M + X is the spontaneous reaction
(c) Ecell = 0.77 V
(d) Ecell = -0.77 V
Correct Answer – (B)
MCQ Questions Class 12 Electrochemistry with Answers
Question 6:
Which of the following is not a good conductor?
(a) Cu
(b) NaCl (aq)
(c) NaCl (molten)
(d) NaCl(s)
Correct Answer – (D)
Question 7:
The standard reduction potentials of Cu2+/Cu and Cu2+/Cu+ are 0.337 and 0.153 respectively. The standard electrode potential of Cu+/Cu half cell is
(a) 0.184 V
(b) 0.827 V
(c) 0.521V
(d) 0.490 V
Correct Answer – (C)
Question 8:
Rust is a mixture of
(a) FeO and Fe (OH)3
(b) FeO and Fe (OH)2
(c) Fe2O3 and Fe (OH)3
(d) Fe3O4 and Fe (OH)3
Correct Answer – (A)
Question 9:
Galvanized iron sheets are coated with
(a) Carbon
(b) Copper
(c) Zinc
(d) Nickel
Correct Answer – (C)
Question 10:
Standard solution of KNO3 is used to make a salt bridge because
(a) Velocity of K+ is greater than that of NO–3
(b) Velocity of NO–3 is greater than that of K+.
(c) Velocity of both K+ and NO–3 are nearly same
(d) KNO3 is highly soluble in water.
Correct Answer – (C)
- NCERT Solutions Class 11 Chemistry Chapter 1 : Some Basic Concepts of Chemistry
- NCERT Solutions Class 11 Chemistry Chapter 2 : Structure Of The Atom
- NCERT Solutions Class 11 Chemistry Chapter 3 : Classification of Elements and Periodicity in Properties
- NCERT Solutions Class 11 Chemistry Chapter 4 : Chemical Bonding and Molecular Structure
- NCERT Solutions Class 11 Chemistry Chapter 5 : States of Matter
- NCERT Solutions Class 11 Chemistry Chapter 6 : Thermodynamics
- NCERT Solutions Class 11 Chemistry Chapter 7 : Equilibrium
- NCERT Solutions Class 11 Chemistry Chapter 8 : Redox Reactions
- NCERT Solutions Class 11 Chemistry Chapter 9 : Hydrogen
- NCERT Solutions Class 11 Chemistry Chapter 10 : The s-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 11 : The p-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 12 : Organic Chemistry: Some Basic Principles and Techniques
- NCERT Solutions Class 11 Chemistry Chapter 13 : Hydrocarbons
- NCERT Solutions Class 11 Chemistry Chapter 14 : Environmental Chemistry