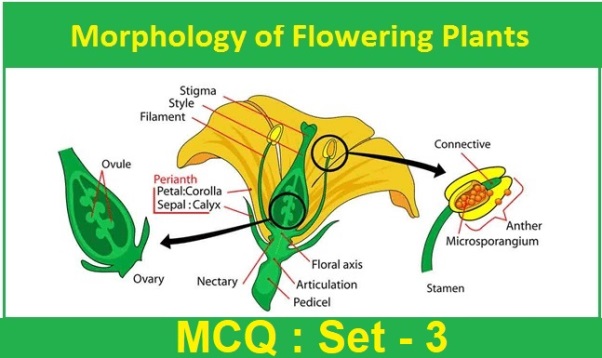
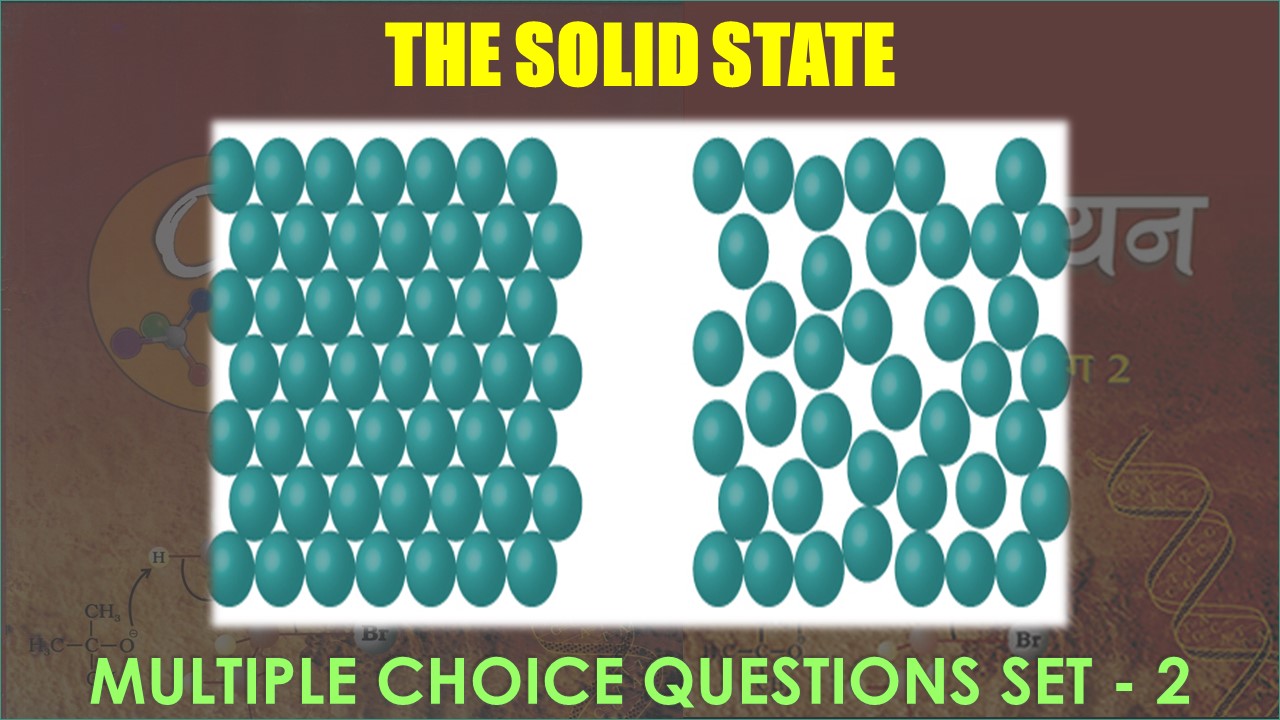
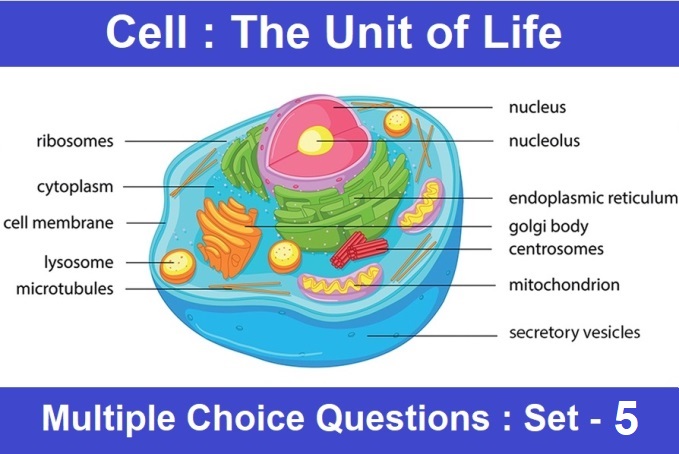
CBSE Class 10 Science Chapter 5 Periodic Classification of Elements Multiple Choice Questions with Answers. MCQ Class 10 Science Periodic Classification of Elements with Answers was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 10 Science Periodic Classification of Elements MCQs with Answers to know their preparation level.
Multiple Choice Questions - Set - 1
Question 1:
Which of the following elements has 2 shells and both are completely filled?
a) Helium
b) Neon
c) Calcium
d) Boron
Correct Answer – (B)
Question 2 :
Element X forms a chloride with the formula XCl2, which is a solid with a high melting point. X would most likely be in the same group of the Periodic Table as
a) Na
b) Mg
c) Al
d) Si
Correct Answer – (B)
Question 3 :
What is the other name for group 18th elements?
a) Noble gases
b) Alkali metals
c) Alkali earth metals
d) Halogens
Correct Answer – (A)
Question 4 :
The electronic configuration of an element M is 2, 8, 4. In modern periodic table, the element M is placed in
a) 4th group
b) 2nd group
c) 14th group
d) 18th group
Correct Answer – (C)
Question 5 :
Which of the following forms the basis of the modern periodic table?
a) Atomic mass
b) Atomic number
c) Number of nucleons
d) All of these
Correct Answer – (B)
Question 6 :
Which group elements are called transition metals?
a) Group number 1 to 2
b) Group number 13 to 18
c) Group number 3 to 12
d) Group number 1 to 8
Correct Answer – (C)
Question 7 :
Which of the following is the most reactive element of the group 17?
a) Oxygen
b) Sodium
c) Fluorine
d) Magnesium
Correct Answer – (C)
Question 8 :
Which of the following is the correct order of the atomic radii of the elements oxygen, fluorine and nitrogen?
a) O < F < N
b) N < F < O
c) O < N < F
d) F < O < N
Correct Answer – (D)
Question 9 :
What happens to the electropositive character of elements on moving from left to right in a periodic table?
a) Increase
b) Decreases
c) First increases than decreases
d) First decreases than increases
Correct Answer – (B)
Question 10 :
How many periods and groups are present in the periodic table?
a) 7 periods and 18 groups
b) 8 periods and 7 groups
c) 7 periods and 7 groups
d) 8 periods and 8 groups
Correct Answer – (A)
- NCERT Solutions Class 10 Science Chapter 1 : Chemical Reactions and Equation
- NCERT Solutions Class 10 Science Chapter 2 : Acids, Bases & Salts
- NCERT Solutions Class 10 Science Chapter 3 : Metals and Non-metals
- NCERT Solutions Class 10 Science Chapter 4 : Carbon And Its Compounds
- NCERT Solutions Class 10 Science Chapter 5 : Periodic Classification of Elements
- NCERT Solutions Class 10 Science Chapter 6 : Life Precesses
- NCERT Solutions Class 10 Science Textbook Download