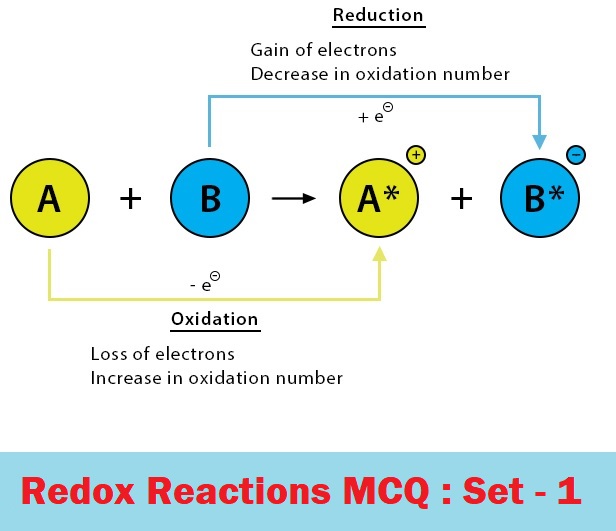
CBSE Class 11 Chemistry Chapter 8 Redox Reactions Multiple Choice Questions with Answers. MCQ Questions Class 11 Chemistry Redox Reactions with Answers was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 11 Chemistry Redox Reactions MCQs with Answers to know their preparation level.
Students who are searching for NCERT MCQ Questions for Class 11 Chemistry Redox Reactions with Answers are compiled here to get good practice on all fundamentals. Know your preparation level on MCQ Questions for Class 11 Chemistry with Answers. You can also verify your answers from our provided MCQ Class 11 Chemistry Redox Reactions with Answers. So, ace up your preparation with MCQ of Chapter 8 Chemistry Objective Questions.
MCQ Questions Class 11 Chemistry Redox Reactions with Answers - Set - 1
Question 1:
The number of moles of KMnO4 reduced by one mole of KI in alkaline medium is
(a) One
(b) Two
(c) Five
(d) One fifth.
Correct Answer – (B)
In alkaline medium the reduction of KMnO4 with KI will takes place as
2 KMnO4 + H2O → 2 KOH + 2 MnO2
KI + 3[O] → KIO3
Hence the overall reaction is
KI + 2KMnO4 + H2O → KIO3 + 2 KOH + 2 MnO2
So, one mole of KI will reduced two moles of KMnO4
Question 2 :
The oxidation state of Cr in Cr (CO)6 is
(a) 0
(b) 2
(c) 2
(d) 6
Correct Answer – (A)
Question 3 :
One mole of N2H4 loses ten moles of electrons to form a new compound A. Assuming that all the nitrogen appears in the new compound, what is the oxidation state of nitrogen in A? (There is no change in the oxidation state of hydrogen.)
(a) -1
(b) -3
(c) +3
(d) +5
Correct Answer – (C)
First to find oxidation number of Nitrogen in N2H4
Oxidation number of H = +1
Let oxidation number of nitrogen be x
2x + 4(1) = 0
2x = -4
x = -2
Each nitrogen atom has -2 oxidation number. So taken both nitrogen atoms in account gives oxidation number -4.
Change in oxidation number of nitrogen on losing 10 mol of electrons (considering no change in oxidation number of hydrogen atoms)
-4 – (-10) = +6
Therefore, oxidation number of 2 nitrogen atoms in compound Y is +6. Hence, oxidation number of each nitrogen atom will be +3 in new compound Y
Question 4 :
If equal volumes of 1M KMnO4 and 1M K2Cr2O7 solutions are allowed to oxidize Fe2+ in acidic medium. The amount of iron oxidized will be:
(a) More with KMnO2
(b) More with K2Cr2O7
(c) Equal with both oxidising agents
(d) Cannot be determined
Correct Answer – (B)
The reason due to which the amount of Fe oxidised will be more with K2Cr2O7 is:
the change in the oxidation state (or number) or n factor is greater with KMnO4
Also, K2Cr2O7 is a very strong oxidising agent and holds the ability to take the electrons but KMnO4 is more stronger than K2Cr2O7
Question 5 :
What is known as Autooxidation?
(a) Formation of H2O by the oxidation of H2O2.
(b) Formation of H2O2 by the oxidation of H2O.
(c) Both (1) and (2) are true
(d) None of the above
Correct Answer – (B)
Explanation:
Autoxidation is any oxidation that occurs in presence of oxygen. The term is usually used to describe the degradation of organic compounds in air (as a source of oxygen). Autoxidation produces hydroperoxides and cyclic organic peroxides. These species can react further to form many products. The process is relevant to many phenomena including aging, paint, and spoilage of foods, degradation of petrochemicals, and the industrial production of chemicals. Autoxidation is important because it is a useful reaction for converting compounds to oxygenated derivatives, and also because it occurs in situations where it is not desired (as in the destructive cracking of the rubber in automobile tires or in rancidification).
Water automatically gets oxidised to hydrogen peroxide
MCQ Questions Class 11 Chemistry Redox Reactions with Answers
Question 6 :
Which of the following processes does not involve oxidation of iron?
(a) Formation of Fe(CO)5 from Fe.
(b) Liberation of H2 from steam by iron at high temperature.
(c) Rusting of iron sheets.
(d) Decolourisation of blue CuSO4 solution by iron
Correct Answer – (A)
Oxidation number of Fe in Fe(CO)5 is zero.
In both Fe and Fe(CO)5, the oxidation state of iron is zero.
3Fe + 4H2O → Fe3O4 + 4H2
team
rusting
Fe → Fe2O3.xH2O
(+3)
CuSO4(aq) + Fe (s) → FeSO4(aq) + Cu(s)
(0) (+2)
Question 7 :
How many millilitres of 0.5 M H2SO4 are needed to dissolve 0.5 g of copper(II)carbonate?
(a) 6.01
(b) 4.5
(c) 8.1
(d) 11.1
Correct Answer – (C)
The volume can be calculated :
N1V1 = N2V2
N1 = Normality of H2SO4 = 0.5 × 2 = 1 N
V1 = Volume of H2SO4
Molar mass of copper(II) carbonate = 123.5 g
N2 = Normality of copper (II) carbonate = (0.5×2)/(123.5) N
V2 = Volume of copper (II) carbonate = 1000 mL
So, after applying the formula,
1 × V1 = (0.5×2)/(123.5)×1000
Hence, V1 = 8.09 mL
= approx. 8.1 mL
Question 8 :
Which of the following processes does not involve either oxidation or reduction?
(a) Formation of slaked lime from quick lime
(b) Heating Mercuric Oxide
(c) Formation of Manganese Chloride from Manganese oxide
(d) Formation of Zinc from Zinc blende
Correct Answer – (A)
Here, in this reaction
CaO + H2O →Ca(OH)2
Oxidation number doesn’t change so its not a redox reaction
Question 9 :
The tendency of an electrode to lose electrons is known as
(a) Electrode Potential
(b) Reduction Potential
(c) Oxidation Potential
(d) E.M.F.
Correct Answer – (C)
The magnitude of the electrode potential of a metal is a measure of its relative tendency to lose or gain electrons. i.e., it is a measure of the relative tendency to undergo oxidation (loss of electrons) or reduction (gain of electrons).
M → Mn+ + ne– (oxidation potential)
Mn+ + ne– → M (reduction potential)
Question 10 :
The oxidation number of Cl in Cl2O7 is
(a) + 7
(b) + 5
(c) + 3
(d) – 7
Correct Answer – (A)
Cl show different oxidation state as -1 to +7 due to vacant d orbital. As oxygen is more electronegative than Cl. Oxygen size is small hence its more electronegative and show -2 oxidation states.
Here Cl2O7 then equation is: 2x + 7 × (-2) = 0
x = +7 hence oxidation state of Cl is +7. I think you get your answer how to find oxidation state
- NCERT Solutions Class 11 Chemistry Chapter 1 : Some Basic Concepts of Chemistry
- NCERT Solutions Class 11 Chemistry Chapter 2 : Structure Of The Atom
- NCERT Solutions Class 11 Chemistry Chapter 3 : Classification of Elements and Periodicity in Properties
- NCERT Solutions Class 11 Chemistry Chapter 4 : Chemical Bonding and Molecular Structure
- NCERT Solutions Class 11 Chemistry Chapter 5 : States of Matter
- NCERT Solutions Class 11 Chemistry Chapter 6 : Thermodynamics
- NCERT Solutions Class 11 Chemistry Chapter 7 : Equilibrium
- NCERT Solutions Class 11 Chemistry Chapter 8 : Redox Reactions
- NCERT Solutions Class 11 Chemistry Chapter 9 : Hydrogen
- NCERT Solutions Class 11 Chemistry Chapter 10 : The s-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 11 : The p-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 12 : Organic Chemistry: Some Basic Principles and Techniques
- NCERT Solutions Class 11 Chemistry Chapter 13 : Hydrocarbons
- NCERT Solutions Class 11 Chemistry Chapter 14 : Environmental Chemistry