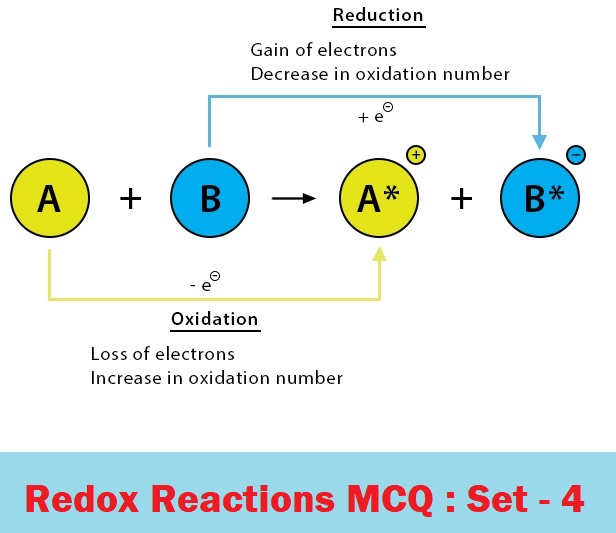
CBSE Class 11 Chemistry Chapter 8 Redox Reactions Multiple Choice Questions with Answers. MCQ Questions Class 11 Chemistry Redox Reactions with Answers was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 11 Chemistry Redox Reactions MCQs with Answers to know their preparation level.
Students who are searching for NCERT MCQ Questions for Class 11 Chemistry Redox Reactions with Answers are compiled here to get good practice on all fundamentals. Know your preparation level on MCQ Questions for Class 11 Chemistry with Answers. You can also verify your answers from our provided MCQ Class 11 Chemistry Redox Reactions with Answers. So, ace up your preparation with MCQ of Chapter 8 Chemistry Objective Questions.
MCQ Questions Class 11 Chemistry Redox Reactions with Answers - Set - 4
Question 1:
The loss of electron is termed as
(a) oxidation
(b) reduction
(c) combustion
(d) neutralization
Correct Answer – (A)
Question 2 :
Which of the following process takes place in oxidation process?
(a) Addition of oxygen
(b) Addition of hydrogen
(c) Removal of oxygen
(d) Addition of chlorine
Correct Answer – (A)
Question 3 :
The oxidation states of sulphur in the anions SO3 2–, S2O4 2– and S2O6 2– follow the order
(a) S2O4 2– < SO3 2– < S2O6 2–
(b) SO3 2– < S2O4 2– < S2O6 2–
(c) S2O4 2– < S2O6 2– < SO3 2–
(d) S2O6 2– < S2O4 2– < SO3 2–
Correct Answer – (A)
Question 4 :
The oxide, which cannot act as a reducing agent is
(a) CO2
(b) ClO2
(c) NO2
(d) SO2
Correct Answer – (A)
Question 5 :
Oxidation state of Fe in Fe3O4 is
(a) 5 4
(b) 4 5
(c) 3 2
(d) 8 3
Correct Answer – (D)
MCQ Questions Class 11 Chemistry Redox Reactions with Answers
Question 6 :
In the reaction given below, identify the species undergoing redox reaction 2Na(s) + H2(g) → 2NaH(s)
(a) Na is reduced and hydrogen is oxidised
(b) Na is oxidised and hydrogen is reduced
(c) Na undergoes oxidation and hydrogen undergoesreduction
(d) Both (b) and (c)
Correct Answer – (D)
Question 7 :
The oxidation state of I in H4IO6 – is
(a) + 1
(b) – 1
(c) + 7
(d) + 5
Correct Answer – (C)
Question 8 :
(I) H2O2 + O3→ H2O + 2O2 (II) H2O2 + Ag2O →2Ag + H2O + O2 Role of hydrogen peroxide in the above reactions is respectively
(a) oxidizing in (I) and reducing in (II)
(b) reducing in (I) and oxidizing in (II)
(c) reducing in (I) and (II)
(d) oxidizing in (I) and (II)
Correct Answer – (C)
Question 9 :
Reaction of sodium thiosulphate with iodine gives
(a) tetrathionate ion
(b) sulphide ion
(c) sulphate ion
(d) sulphite ion.
Correct Answer – (A)
Question 10 :
Hot concentrated sulphuric acid is a moderately strong oxidizing agent. Which of the followingreactions does not show oxidizing behaviour?
(a) Cu + 2H2SO4 → CuSO4 + SO2 + 2H2O
(b) S + 2H2SO4 → 3SO2 + 2H2O
(c) C + 2H2SO4 → CO2 + 2SO2 + 2H2O
(d) CaF2 + H2SO4 → CaSO4 + 2HF
Correct Answer – (D)
- NCERT Solutions Class 11 Chemistry Chapter 1 : Some Basic Concepts of Chemistry
- NCERT Solutions Class 11 Chemistry Chapter 2 : Structure Of The Atom
- NCERT Solutions Class 11 Chemistry Chapter 3 : Classification of Elements and Periodicity in Properties
- NCERT Solutions Class 11 Chemistry Chapter 4 : Chemical Bonding and Molecular Structure
- NCERT Solutions Class 11 Chemistry Chapter 5 : States of Matter
- NCERT Solutions Class 11 Chemistry Chapter 6 : Thermodynamics
- NCERT Solutions Class 11 Chemistry Chapter 7 : Equilibrium
- NCERT Solutions Class 11 Chemistry Chapter 8 : Redox Reactions
- NCERT Solutions Class 11 Chemistry Chapter 9 : Hydrogen
- NCERT Solutions Class 11 Chemistry Chapter 10 : The s-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 11 : The p-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 12 : Organic Chemistry: Some Basic Principles and Techniques
- NCERT Solutions Class 11 Chemistry Chapter 13 : Hydrocarbons
- NCERT Solutions Class 11 Chemistry Chapter 14 : Environmental Chemistry