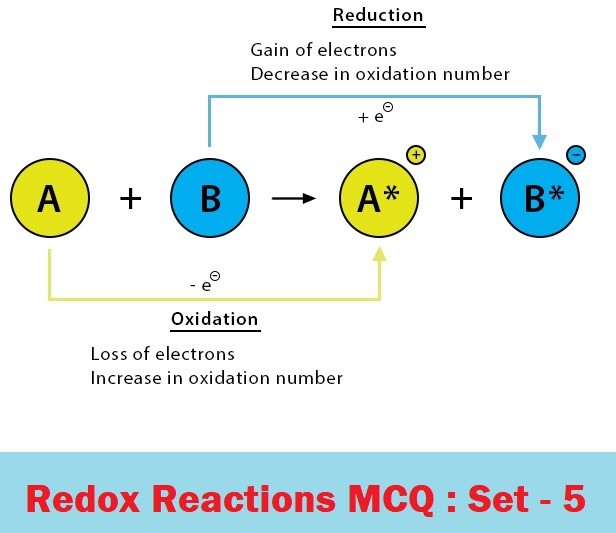
CBSE Class 11 Chemistry Chapter 8 Redox Reactions Multiple Choice Questions with Answers. MCQ Questions Class 11 Chemistry Redox Reactions with Answers was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 11 Chemistry Redox Reactions MCQs with Answers to know their preparation level.
Students who are searching for NCERT MCQ Questions for Class 11 Chemistry Redox Reactions with Answers are compiled here to get good practice on all fundamentals. Know your preparation level on MCQ Questions for Class 11 Chemistry with Answers. You can also verify your answers from our provided MCQ Class 11 Chemistry Redox Reactions with Answers. So, ace up your preparation with MCQ of Chapter 8 Chemistry Objective Questions.
MCQ Questions Class 11 Chemistry Redox Reactions with Answers - Set - 5
Question 1:
Co(s) + Cu2+ (aq)→Co2+ (aq) + Cu(s) The above reaction is
(a) oxidation reaction
(b) reduction reaction
(c) redox reaction
(d) None of these
Correct Answer – (C)
Question 2 :
Which of the following is the correct representative of stock notation for auric chloride?
(a) Au(III)Cl3
(b) Au(II)Cl2
(c) Au(I)Cl2
(d) None of these
Correct Answer – (A)
Question 3 :
In which of the following compounds oxygen has highest oxidation state and in which it has lowest oxidation state? OF2, H2O2, KO2, O2F2
(a) Highest = KO2, lowest = H2O2
(b) Highest = OF2, lowest = K2O2
(c) Highest = OF2, lowest = KO2
(d) Highest = KO2, lowest = H2O2
Correct Answer – (C)
Question 4 :
The correct order of electron releasing tendency of the metals Cu, Zn and Ag is in the order:
(a) Cu > Zn > Ag
(b) Zn > Ag > Cu
(c) Ag > Zn > Cu
(d) Zn > Cu > Ag
Correct Answer – (D)
Question 5 :
Which one of the following reaction involves oxidationreduction ?
(a) H2 +Br2 →2HBr
(b) NaBr + HCl → NaCl + HBr
(c) HBr + AgNO 3 → AgBr + HNO 3
(d) 2NaOH + H2SO4 → Na 2SO4 + 2H2O
Correct Answer – (A)
MCQ Questions Class 11 Chemistry Redox Reactions with Answers
Question 6 :
In reaction, 4Na + O2 → 2Na2O, sodium behaves as
(a) oxidising agent
(b) reducing agent
(c) Both (a) and (b)
(d) None of these
Correct Answer – (B)
Question 7 :
‘Oxidation number of H in NaH, CaH2 and LiH, respectively is
(a) +1, +1, –1
(b) –1, +1, + 1
(c) +1, + 1, + 1
(d) –1,–1, –1
Correct Answer – (D)
Question 8 :
What is the oxidation number of elements in the free or in the uncombined state ?
(a) +1
(b) 0
(c) +2
(d) –1
Correct Answer – (B)
Question 9 :
When a strip of metallic zinc is placed in an aqueous solution of copper nitrate the blue colour of the solutiondisappear due to formation of
(a) Cu2+
(b) Zn2+
(c) ZnS
(d) Cus
Correct Answer – (B)
Question 10 :
In the following reaction 4P + 3KOH+ 3H2O→3KH2PO2 + PH3
(a) phosphorus is both oxidised and reduced.
(b) only phosphorus is reduced.
(c) phosphorus is not oxidised
(d) None of these
Correct Answer – (A)
- NCERT Solutions Class 11 Chemistry Chapter 1 : Some Basic Concepts of Chemistry
- NCERT Solutions Class 11 Chemistry Chapter 2 : Structure Of The Atom
- NCERT Solutions Class 11 Chemistry Chapter 3 : Classification of Elements and Periodicity in Properties
- NCERT Solutions Class 11 Chemistry Chapter 4 : Chemical Bonding and Molecular Structure
- NCERT Solutions Class 11 Chemistry Chapter 5 : States of Matter
- NCERT Solutions Class 11 Chemistry Chapter 6 : Thermodynamics
- NCERT Solutions Class 11 Chemistry Chapter 7 : Equilibrium
- NCERT Solutions Class 11 Chemistry Chapter 8 : Redox Reactions
- NCERT Solutions Class 11 Chemistry Chapter 9 : Hydrogen
- NCERT Solutions Class 11 Chemistry Chapter 10 : The s-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 11 : The p-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 12 : Organic Chemistry: Some Basic Principles and Techniques
- NCERT Solutions Class 11 Chemistry Chapter 13 : Hydrocarbons
- NCERT Solutions Class 11 Chemistry Chapter 14 : Environmental Chemistry