CBSE Class 11 Chemistry Chapter 5 States of Matter Multiple Choice Questions with Answers. MCQ Questions Class 11 Chemistry States of Matter with Answers was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 11 Chemistry States of Matter MCQs with Answers to know their preparation level.
Students who are searching for NCERT MCQ Questions for Class 11 Chemistry States of Matter with Answers are compiled here to get good practice on all fundamentals. Know your preparation level on MCQ Questions for Class 11 Chemistry with Answers. You can also verify your answers from our provided MCQ Class 11 Chemistry States of Matter with Answers. So, ace up your preparation with MCQ of Chapter 5 Chemistry Objective Questions.
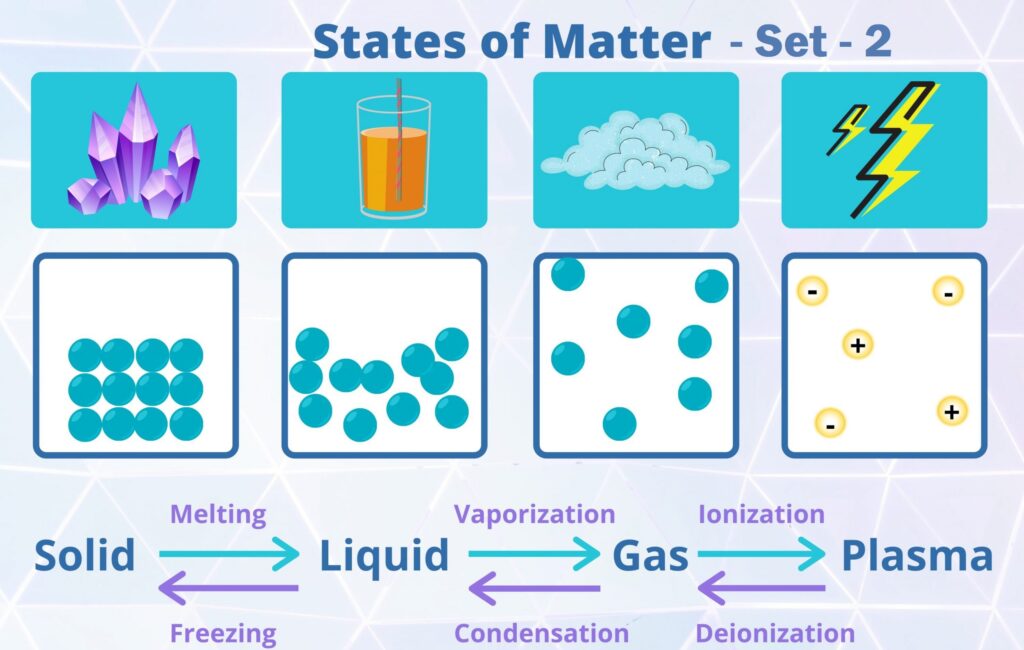
MCQ Questions Class 11 Chemistry States of Matter with Answers - Set - 2
Question 1:
When you heat a sample of gas, what happens to the particles that make up the gas?
(a) The particles move faster.
(b) The particles break apart
(c) The particles get smaller
(d) The particles become more dense
Correct Answer – (A)
There is a great deal of empty space between particles, which have a lot of kinetic energy. The particles move very fast and collide into one another when the gas is heated up, causing them to diffuse, or spread out, until they are evenly distributed throughout the volume of the container
Question 2 :
The law, which states that at constant temperature, the volume of a given mass of gas is inversely proportional is pressure, is known as:
(a) Boyles law
(b) Charles law
(c) Combine gas law
(d) Avogadros law
Correct Answer – (A)
In 1662 Robert Boyle studied the relationship between volume and pressure of a gas of fixed amount at constant temperature. He observed that volume of a given mass of a gas is inversely proportional to its pressure at a constant temperature. Boyles law, published in 1662, states that, at constant temperature, the product of the pressure and volume of a given mass of an ideal gas in a closed system is always constant. It can be verified experimentally using a pressure gauge and a variable volume container. It can also be derived from the kinetic theory of gases: if a container, with a fixed number of molecules inside, is reduced in volume, more molecules will strike a given area of the sides of the container per unit time, causing a greater pressure.
A statement of Boyles law is as follows:
The volume of a given mass of a gas is inversely related to pressure when the temperature is constant.
V ∝ (1/P) meaning “Volume is inversely proportional to Pressure”, or
where P is the pressure, and V is the volume of a gas, and k1 is the constant in this equation
Question 3 :
Falling drop of water is spherical due to:
(a) Hydrogen Bonding
(b) Surface Tension
(c) Capillary Action
(d) Vlscosity
Correct Answer – (B)
Raindrops take up the spherical shape due to the surface tension of water which is caused due to the tendency of water molecules to stick together. The spherical shape is having the least possible surface area due to which it can resist any of the external force in the atmosphere
Question 4 :
The rise or fall of a liquid within a tube of small bore is called:
(a) Surface Tension
(b) Capillary Action
(c) Viscosity
(d) Formation of Curvature
Correct Answer – (B)
Explanation:
Capillarity, rise or depression of a liquid in a small passage such as a tube of small cross-sectional area, like the spaces between the fibres of a towel or the openings in a porous material. Capillarity is not limited to the vertical direction. Water is drawn into the fibres of a towel, no matter how the towel is oriented.
Liquids that rise in small-bore tubes inserted into the liquid are said to wet the tube, whereas liquids that are depressed within thin tubes below the surface of the surrounding liquid do not wet the tube. Water is a liquid that wets glass capillary tubes; mercury is one that does not. When wetting does not occur, capillarity does not occur.
Capillarity is the result of surface, or interfacial, forces. The rise of water in a thin tube inserted in water is caused by forces of attraction between the molecules of water and the glass walls and among the molecules of water themselves. These attractive forces just balance the force of gravity of the column of water that has risen to a characteristic height. The narrower the bore of the capillary tube, the higher the water rises. Mercury, conversely, is depressed to a greater degree, the narrower the bore
Question 5 :
How many of the know elements exist as gases at 25°C?
(a) 9
(b) 11
(c) 12
(d) 15
Correct Answer – (B)
MCQ Questions Class 11 Chemistry States of Matter with Answers
Question 6 :
If helium and methane are allowed to diffuse out of the container under the similar conditions of temperature and pressure, then the ratio of rate of diffusion of helium to methane is:
(a) 2 : 1
(b) 1 : 2
(c) 3 : 5
(d) 4 : 1
Correct Answer – (A)
Question 7 :
The rates of diffusion of gases are inversely proportional to square root of their densities . This statement refers to :
(a) Daltons Law
(b) Grahams Law
(c) Avogadros Law
(d) None of the Above
Correct Answer – (B)
Grahams law states that the rate of diffusion of a gas is inversely proportional to the square root of its molecular weight. In the same conditions of temperature and pressure, the molar mass is proportional to the mass density. Therefore the rate of diffusion of different gases is inversely proportional to the square root of their mass densities
Question 8 :
The theory which explains that gases consist of molecules, which are in rapid option is known as:
(a) Daltons Atomic Theory
(b) Bohrs Theory
(c) Rutherfords Atomic Theory
(d) Kinetic Molecular Theory
Correct Answer – (D)
Explanation:
The kinetic molecular theory (KMT) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. This theory is based on the following five postulates described here. (Note: The term “molecule” will be used to refer to the individual chemical species that compose the gas, although some gases are composed of atomic species, for example, the noble gases.)
Gases are composed of molecules that are in continuous motion, travelling in straight lines and changing direction only when they collide with other molecules or with the walls of a container.
The molecules composing the gas are negligibly small compared to the distances between them.
The pressure exerted by a gas in a container results from collisions between the gas molecules and the container walls.
Gas molecules exert no attractive or repulsive forces on each other or the container walls; therefore, their collisions are elastic (do not involve a loss of energy).
The average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas
Question 9 :
The states of matter having no definite shape but definite volume:
(a) Gas
(b) Liquid
(c) Solid
(d) None of the Above
Correct Answer – (B)
In a liquid, particles will flow or glide over one another, but stay toward the bottom of the container. The attractive forces between particles are strong enough to hold a specific volume but not strong enough to keep the molecules sliding over each other
Question 10 :
If 20cm³ gas at 1 atm. is expanded to 50 cm³ at constant T, then what is the final pressure
(a) 20 × 50
(b) 50 × 120
(c) 1 × 120 × 50
(d) None of these
Correct Answer – (A)
At constant T, P1V1 = P2V2
1 × 20 = P2 × 50;
P2 = (20/50) X 1
- NCERT Solutions Class 11 Chemistry Chapter 1 : Some Basic Concepts of Chemistry
- NCERT Solutions Class 11 Chemistry Chapter 2 : Structure Of The Atom
- NCERT Solutions Class 11 Chemistry Chapter 3 : Classification of Elements and Periodicity in Properties
- NCERT Solutions Class 11 Chemistry Chapter 4 : Chemical Bonding and Molecular Structure
- NCERT Solutions Class 11 Chemistry Chapter 5 : States of Matter
- NCERT Solutions Class 11 Chemistry Chapter 6 : Thermodynamics
- NCERT Solutions Class 11 Chemistry Chapter 7 : Equilibrium
- NCERT Solutions Class 11 Chemistry Chapter 8 : Redox Reactions
- NCERT Solutions Class 11 Chemistry Chapter 9 : Hydrogen
- NCERT Solutions Class 11 Chemistry Chapter 10 : The s-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 11 : The p-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 12 : Organic Chemistry: Some Basic Principles and Techniques
- NCERT Solutions Class 11 Chemistry Chapter 13 : Hydrocarbons
- NCERT Solutions Class 11 Chemistry Chapter 14 : Environmental Chemistry