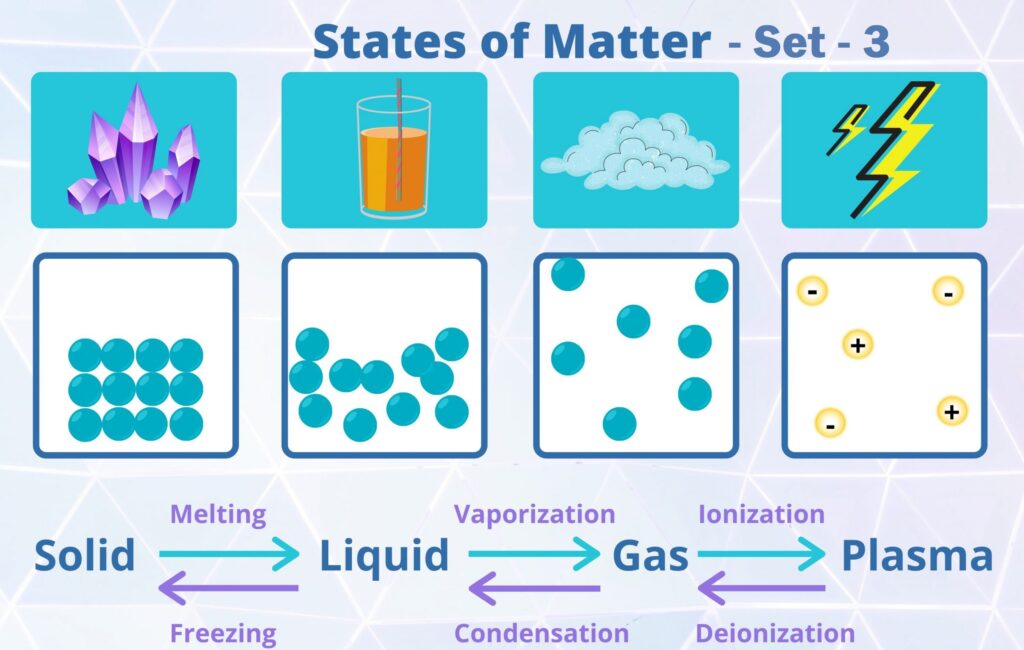
CBSE Class 11 Chemistry Chapter 5 States of Matter Multiple Choice Questions with Answers. MCQ Questions Class 11 Chemistry States of Matter with Answers was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 11 Chemistry States of Matter MCQs with Answers to know their preparation level.
Students who are searching for NCERT MCQ Questions for Class 11 Chemistry States of Matter with Answers are compiled here to get good practice on all fundamentals. Know your preparation level on MCQ Questions for Class 11 Chemistry with Answers. You can also verify your answers from our provided MCQ Class 11 Chemistry States of Matter with Answers. So, ace up your preparation with MCQ of Chapter 5 Chemistry Objective Questions.
MCQ Questions Class 11 Chemistry States of Matter with Answers - Set - 3
Question 1:
For an ideal gas, number of moles per litre in terms of its pressure P, gas constant R and temperature T is
(a) PT/R
(b) PRT
(c) P/RT
(d) RT/P
Correct Answer – (A)
Question 2 :
0.5 mole of each of H2, SO2 and CH4 are kept in a container. A hole was made in the container After 3 hours, the order of partial pressures in the container will be
(a) PsO2 > PCH4 > PH2
(b) PH2 > PSO2 > PCH4
(c) PCH4 > Pso2 > PH2
(d) PH2 > PcH4 > Pso2
Correct Answer – (A)
Question 3 :
What is the molar mass of C gas whose density is 1.5 g L-1 at 27°C and 1 atm pressure [R = 0.08 L atm K-1 mol-1].
(a) 360
(b) 720
(c) 36
(d) 18
Correct Answer – (C)
Question 4 :
The density of a gas is 4 times that of Y. If the molar mass M, that of Y is
(a) 2M
(b) M/2
(c) 4M
(d) M/4
Correct Answer – (D)
Question 5 :
Rate of effusion of a gas is
(a) Directly proportional to its density
(b) Directly proportional to its molar mass
(c) Directly proportional to the square root of its mass
(d) Inversely proportional to the square root of its molar mass.
Correct Answer – (D)
MCQ Questions Class 11 Chemistry States of Matter with Answers
Question 6 :
If r.sm.s. speed of gaseous molecules is x cm sec-1 at a pressure of p atm, then r.m.s. speed at a pressure of 2p atm and constant pressure will be
(a) x
(b) 2x
(c) 4x
(d) x/4
Correct Answer – (A)
Question 7 :
At STP the density of nitrogen monoxide is
(a) 3.0 g L-1
(b) 30 gL-1
(c) 1.34 g L-1
(d) 2.68 gL-1
Correct Answer – (C)
Question 8 :
The pressure of 2 moles of an ideal gas at 273°C occupying a volume of 44.8 L is
(a) 2 atm
(b) 1 atm
(c) 3 atm
(d) 4 atm
Correct Answer – (A)
Question 9 :
A gas diffuses 1/5 times as fast as hydrogen. Its molar mass is
(a) 25
(b) 50
(c) 25√2
(d) 50√2
Correct Answer – (B)
Question 10 :
The density of neon will be highest at
(a) STP
(b) 0°C and 2 atm
(c) 273°C, 1 atm
(d) 273°C, 0.5 atm
Correct Answer – (B)
- NCERT Solutions Class 11 Chemistry Chapter 1 : Some Basic Concepts of Chemistry
- NCERT Solutions Class 11 Chemistry Chapter 2 : Structure Of The Atom
- NCERT Solutions Class 11 Chemistry Chapter 3 : Classification of Elements and Periodicity in Properties
- NCERT Solutions Class 11 Chemistry Chapter 4 : Chemical Bonding and Molecular Structure
- NCERT Solutions Class 11 Chemistry Chapter 5 : States of Matter
- NCERT Solutions Class 11 Chemistry Chapter 6 : Thermodynamics
- NCERT Solutions Class 11 Chemistry Chapter 7 : Equilibrium
- NCERT Solutions Class 11 Chemistry Chapter 8 : Redox Reactions
- NCERT Solutions Class 11 Chemistry Chapter 9 : Hydrogen
- NCERT Solutions Class 11 Chemistry Chapter 10 : The s-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 11 : The p-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 12 : Organic Chemistry: Some Basic Principles and Techniques
- NCERT Solutions Class 11 Chemistry Chapter 13 : Hydrocarbons
- NCERT Solutions Class 11 Chemistry Chapter 14 : Environmental Chemistry