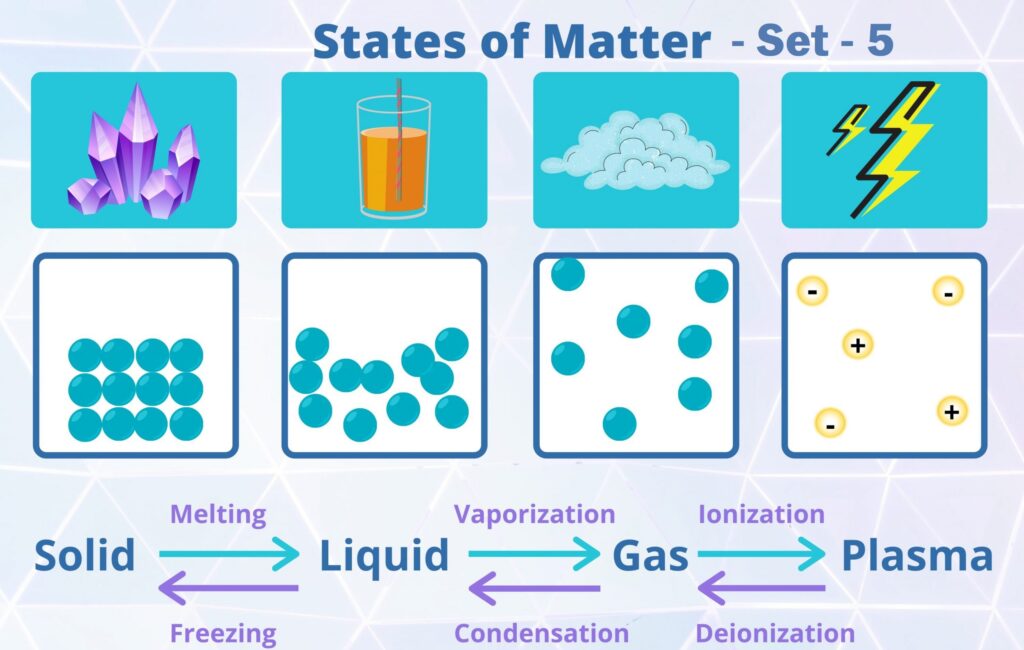
CBSE Class 11 Chemistry Chapter 5 States of Matter Multiple Choice Questions with Answers. MCQ Questions Class 11 Chemistry States of Matter with Answers was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 11 Chemistry States of Matter MCQs with Answers to know their preparation level.
Students who are searching for NCERT MCQ Questions for Class 11 Chemistry States of Matter with Answers are compiled here to get good practice on all fundamentals. Know your preparation level on MCQ Questions for Class 11 Chemistry with Answers. You can also verify your answers from our provided MCQ Class 11 Chemistry States of Matter with Answers. So, ace up your preparation with MCQ of Chapter 5 Chemistry Objective Questions.
MCQ Questions Class 11 Chemistry States of Matter with Answers - Set - 5
Question 1:
The density of a gas is 1.964 g dm-3 at 273 K and 76 cm Hg. The gas is
(a) CH4
(b) C2H6
(c) CO2
(d) Xe
Correct Answer – (C)
Question 2 :
When the temperature is increased, surface tension of water
(a) increases
(b) decreases
(c) remains constant
(d) shows irregular behaviour
Correct Answer – (B)
Question 3 :
The compressibility of a gas is less than unity at STP. Therefore
(a) Vm > 22.4 litres
(b) Vm < 22.4 litres
(c) Vm = 22.4 litres
(d) Vm = 44.8 litres
Correct Answer – (C)
Question 4 :
4.4 g of a gas at STP occupies a volume of 2.24 L, the gas can be
(a) O2
(b) CO
(c) NO2
(d) CO2
Correct Answer – (D)
Question 5 :
A 4 : 1 mixture of helium and methane is contained in a vessel at 10 bar pressure. Due to a hole in the vessel, the gas mixture leaks out. The composition of the mixture effusing out initially is
(a) 8 : 1
(b) 8 : 3
(c) 4 : 1
(d) 1 : 1
(e) 2 : 1
Correct Answer – (A)
MCQ Questions Class 11 Chemistry States of Matter with Answers
Question 6 :
The van der Waals equation reduces itself to the ideal gas equation at
(a) high pressure and low temperature
(b) low pressure and low temperature
(c) low pressure and high temperature
(d) low pressure and low temperature
Correct Answer – (C)
Question 7 :
If the four tubes of a car are filled to the same pressure with N2, O2, H2 and Ne separately, then which one will be filled first?
(a) N2
(b) O2
(c) H2
(d) Ne
Correct Answer – (C)
Question 8 :
Gas equation PV = nRT is obeyed by
(a) Only isothermal process
(b) Only adiabatic process
(c) Both (a) and (b)
(d) None of these
Correct Answer – (C)
Question 9 :
The temperature and pressure of 4 dm³ of CO2 gas are doubled. Then the volume of CO2 gas would be
(a) 2 dm³
(b) 3 dm³
(c) 4 dm³
(d) 8 dm³
Correct Answer – (C)
Question 10 :
If a mixture of CO and N2 in equal amount have total 1 atm pressure, then partial pressure of N2 in the mixture is
(a) 1 atm
(b) 0.50 atm
(c) 2 atm
(d) 3 atm
Correct Answer – (B)
- NCERT Solutions Class 11 Chemistry Chapter 1 : Some Basic Concepts of Chemistry
- NCERT Solutions Class 11 Chemistry Chapter 2 : Structure Of The Atom
- NCERT Solutions Class 11 Chemistry Chapter 3 : Classification of Elements and Periodicity in Properties
- NCERT Solutions Class 11 Chemistry Chapter 4 : Chemical Bonding and Molecular Structure
- NCERT Solutions Class 11 Chemistry Chapter 5 : States of Matter
- NCERT Solutions Class 11 Chemistry Chapter 6 : Thermodynamics
- NCERT Solutions Class 11 Chemistry Chapter 7 : Equilibrium
- NCERT Solutions Class 11 Chemistry Chapter 8 : Redox Reactions
- NCERT Solutions Class 11 Chemistry Chapter 9 : Hydrogen
- NCERT Solutions Class 11 Chemistry Chapter 10 : The s-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 11 : The p-Block Elements
- NCERT Solutions Class 11 Chemistry Chapter 12 : Organic Chemistry: Some Basic Principles and Techniques
- NCERT Solutions Class 11 Chemistry Chapter 13 : Hydrocarbons
- NCERT Solutions Class 11 Chemistry Chapter 14 : Environmental Chemistry